Pulsed Lavage System
Your current position:Home > Products > Pulse Lavage System > Pulsed Lavage System > Pulse Lavage SystemThe application of Pulsed lavage has been clinically proven to be essential for both orthopaedic procedures and wound debridement. In arthroplasty, bone bed cleaning with a Pulsed Lavage is a key aspect to the long-term survival of the prosthesis. In wound debridement, it effectively removes more than 90% of the necrotic tissues, contaminant and bacteria, consequently reduces infections rates. The DPLS is designed accordingly to fulfill these functions. This ergonomic, low noise, light weight system offers both a powerful gear for orthopaedic applications and a gentle gear for soft tissue debridement. Concurrent suction and irrigation efficiently does its job without flooding the field.
The system is delivered sterile and for single use
- Product details
Pulse Lavage System
Reference Code: HTF0101
1. Device name
1) Device name: Disposable Electronically Pulsed Lavage Suction Apparatus
2. Indications
1) This device is used together with normal sterile saline or other applicable solutions, vacuum extractor. Vacuum extractor is not a must.
2) This device use for open wound cleaning, soft tissue cleaning, surgical operation location cleaning.
3) This device is suitable to be used in operation room, emergency room and treatment room.
3. Features
1) This device is easy to use and portable with significant cleansing effect.
2) This device is powered by 8 pieces of 1.5V DC mercury-free AA alkaline batteries.
3) This device is portable and can be installed and handled easily.
4) This device is suitable for any kind of open wound cleansing.
5) This device cleans the wound completely with proper pressure.
6) This device avoids cross contamination with its disposability.
7) This device removes waste solution with suction, when the waste pipe is connected with vacuum extractor.
4. Elements
1) This device is a portable, internal battery-powered
2) This device consists of DC motor, compression pumps, handpiece, enclosure, battery, and nozzle.
3) When squeeze the trigger power is on. When power is turned on, DC motor drives the compression pumps in reciprocating motion, engender cavity volume changes, thus it can breathe in and out the solution. The power is 12V DC battery. There have 6V DC ,12V DC and stop tap position. Different nozzles can be fitted on during operation.
5. Components
1) This device consists of Handpiece (1) 【Including DC motor, Compression pumps, Trigger (1.1), Nozzle lock (1.2), Enclosure.】, Nozzle (2) 【Including Short nozzle(2a), Long nozzle(2b) and other nozzles.】, Waste pipe (3) 【Including Clamp (3.1), Connecting pipe (3.2).】, Irrigation pipe (4) 【Including Clamp (3.1), Luer fitting (4.2).】,Battery bag(5), Power wire(6).
2) The Vacuum extractor (7) and Irrigation bag (8) are not including in this device, but prepared by medical institution.
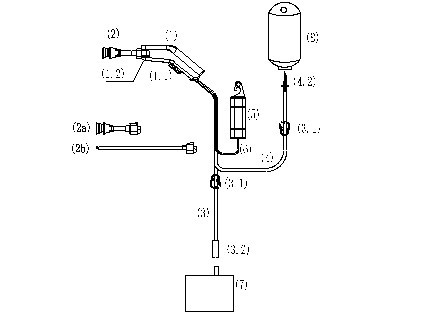
Device structure diagram
6. Operating instructions
1) Inspect the package. Do not use if inner package is damaged.
2) See user manual and attached documents.
3) Open the package, and install Nozzle (2) to Handpiece (1). Press down the Nozzle lock (1.2) to lock the Nozzle (2).
4) Connect the Waste pipe (3) with Vacuum extractor (7) (if prepared). Vacuum extractor is not a must.
5) Use the fitting (4.2) to spike the Irrigation bag (8).
6) Hang up or place the Battery bag (5).
7) Use the Nozzle (2) to aim the wound. Squeeze Trigger (1.1) to activate the device and start irrigation. The Trigger (1.1) have 6V DC,12V DC and stop tap position.
8) Slide Clamp (3.1) can control the liquid flow in the Irrigation pipe (4) and Waste pipe (3). Please lock the Clamp (3.1), when stop to operating the device.
9) It can suck the waste solution concurrently while spraying or after, if Vacuum extractor (7) is prepared.
10) Press Nozzle lock (1.2) upward first, when withdraw the Nozzle (2).
7. Specification
1) Powered by 8 pieces of 1.5V DC mercury-free AA alkaline batteries (total 12V DC).
2) Handpiece dimensions: 185mm*55mm*40mm (Long* Wide* High).
3) Net weight: less than 0.80kg.
4) Flow rate: more than0.65L/minute.
5) Decibel: less than 75dB (A).
6) Maximum irrigation length: not less than 2 meter.
7) Solution form: fog-like.
8) This device is sterilized using ethylene oxide before delivery. Sterilization validity is two years.
8. Classification
1) Internal electrical power source equipment.
2) Type B applied part.
3) IPX0.
4) Not category AP / APG equipment.
5) Mode of operation: Total operation time of each device shall not exceed 20 minutes.
9. Contraindications
No.
10. Warnings
1) This device is only for qualified professionals operation.
2) This device should not be used after the expiry date shown on the pack.
3) This device is for single patient use only. Do not reuse the device.
4) Prior to each use, inspect the package. Do not use if inner package is damaged.
5) This device is not evaluated as category AP or APG equipment. This device is not suitable for use in the presence of flammable anesthetic mixtures with air or oxygen or nitrous oxide.
11. Precautions
1) This device sterilized using ethylene oxide before delivery. Sterilization validity is two years.
2) This device contains alkaline batteries. Follow current regulations to properly recycle or dispose of this unit after use.
3) Total operation time of each device shall not exceed 45 minutes.
4) This device is maintenance free and doesn’t require routine maintenance.
5) This device doesn’t contain user repairable parts.
6) This device is for single patient use only. There is no cleaning or sterilization requirement.
12. Adverse effect
No.
13. Maintenance, Cleaning And Sterilization
1) This device is maintenance free and doesn’t require routine maintenance.
2) This device doesn’t contain user repairable parts.
3) This device is for single patient use only. There is no cleaning or sterilization requirement.
14. Environmental Requirements
1) Storage conditions:
This device should be stored in non-corrosive gases, cool, dry, and ventilated place.
Temperature:-40℃~+55℃.
Humidity: ≤95%RH.
Air pressure: 50kPa~106kPa.
2) Operating conditions:
Temperature:10℃~40℃.
Humidity: ≤80%RH.
Air pressure: 86kPa~106kPa.





